Table of Contents
Biomolecules Definition
Any molecule that is generated by living beings referred as Biomolecules. A molecule is an electrically neutral collection of atoms that may live alone in a free state while retaining its distinctive characteristics in physics and chemistry. The atoms that make up the molecule might be of the same sort (as in the oxygen molecule, which is made up of two oxygen atoms) or different kinds (as in the hydrogen molecule, which is made up of two hydrogen atoms) (such as water molecule made up of oxygen and hydrogen).
A molecule is a word used less strictly in biology, particularly in biochemistry, to refer to any minute particle such as proteins, carbohydrates, DNA, and so on.
What are Biomolecules?
Any molecule generated by living creatures is referred to as a biomolecule. As a result, the vast majority of them are organic compounds. Polysaccharides, amino acids and proteins, nucleic acids (DNA and RNA), and lipids are the four primary types of macromolecules found in and generated by living organisms.
As a result, many biomolecules are polymers. Polymerization produces a polymer, which is a molecule made up of multiple repeating units (monomers) or protomers. Organic chemicals make up the majority of these biomolecules.
Being “organic” implies they include carbon atoms that are covalently linked to other atoms, particularly Carbon-Carbon (C-C) and Carbon-Hydrogen (C-H). Carbon, hydrogen, oxygen, and nitrogen are the four main elements that make up carbon.
Types of Biomolecules
i. Nucleic Acid
A nucleic acid is a biomolecule that is made up of monomeric nucleotide units. Phosphoric acid, sugar (5-carbon), and nitrogenous base make up each nucleotide in turn. 3′, 5′ phosphodiester linkages connect the nucleotide chains of a nucleic acid.
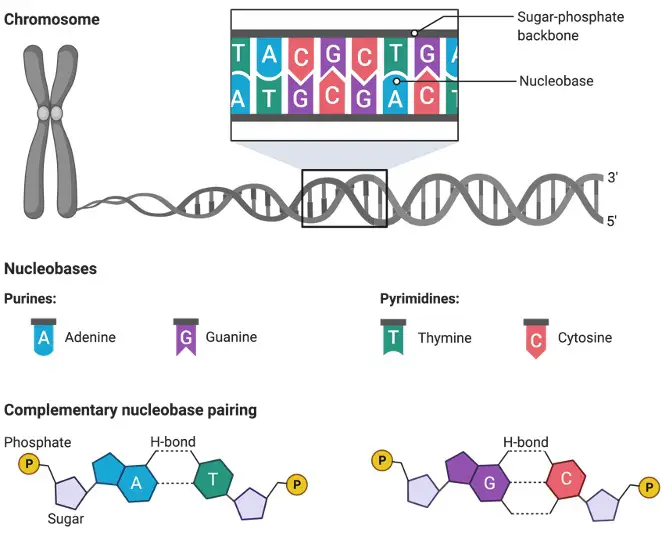
Nucleic acids are DNA or RNA molecules that hold the genetic information required for all biological processes and inheritance. A nucleoside, on the other hand, is a biomolecule that is created when a nucleobase is linked to a ribose or deoxyribose.
Cytidine, uridine, adenosine, guanosine, and thymidine are among examples. Phosphorylated nucleosides are converted to nucleotides. Nucleotides, in addition to acting as a structural unit of nucleic acids, can also be used as a source of chemical energy (e.g. adenosine triphosphate or ATP).
Some enzymatic processes may require nucleotides as cofactors (e.g. flavin adenine dinucleotide or FAD, nicotinamide adenine dinucleotide phosphate or NADP). DNA, or deoxyribonucleic acid, is a double-stranded nucleic acid that contains a living organism’s genetic information.
It’s made up of two strands twisted together to form a helix. Each strand is made up of alternating phosphate and pentose sugar (2-deoxyribose), with a nitrogenous base (adenine, thymine, guanine, or cytosine) linked to the sugar.
DNA is required for an organism’s cell development, division, and function. RNA, also known as ribonucleic acid, is a single-stranded nucleic acid made up of repeated nucleotide units of ribose sugar, a phosphate group, and a nitrogenous base.
It is made up of a long linear chain of nucleotides, each of which has a sugar, phosphate group, and nitrogenous base. The sugar backbone is ribose (deoxyribose in DNA) and the bases are adenine, guanine, cytosine, and uracil, which distinguishes it from a DNA molecule.
DNA and RNA are biopolymers made up of mononucleotide repeating units that are mostly made up of carbon, hydrogen, oxygen, nitrogen, and phosphorus.
ii. Proteins
Proteins are biomolecules made up of amino acids linked by peptide bonds. The basic amino group (NH2), the acidic carboxylic group (COOH), a hydrogen atom, and an organic side group (R) linked to the carbon atom make up an amino acid.
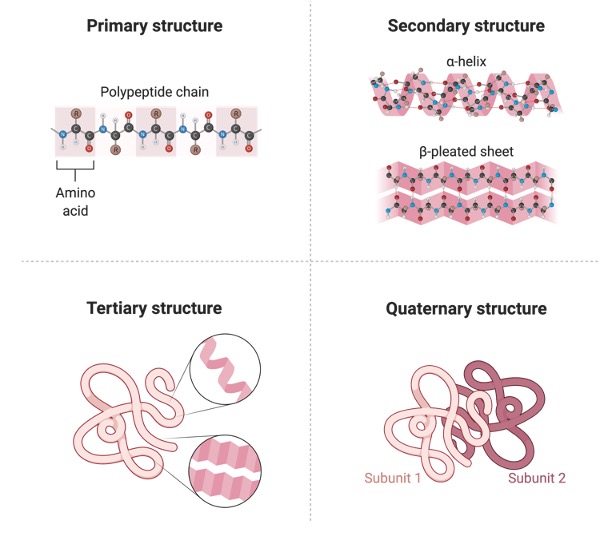
As a result, its fundamental formula is NH2CHRCOOH. The classification of amino acids is based on whether they are non-essential or essential. In the body, non-essential (or dispensed) amino acids are produced. Essential (or necessary) amino acids are those that cannot be produced by the body and must be acquired from diet.
A hundred natural amino acids have been discovered. Twenty of these are necessary for the formation of a protein. Proteins are required for a variety of biological tasks, including structural material (e.g. keratin), enzymes, transporters (e.g. haemoglobin), antibodies, and gene regulators.
iii. Carbohydrates
Carbohydrates (sugars) are the most common of the major biomolecule classes. The majority of carbohydrates have the general formula Cn (H2O) n, from whence they get their name, which means carbon hydrates.
However, not all carbs follow this formula, and some have a structure that differs from the norm. Chemically, they are aldehydes or ketones with a large number of hydroxyl groups attached to each carbon atom that is not part of the aldehyde or ketone functional group.
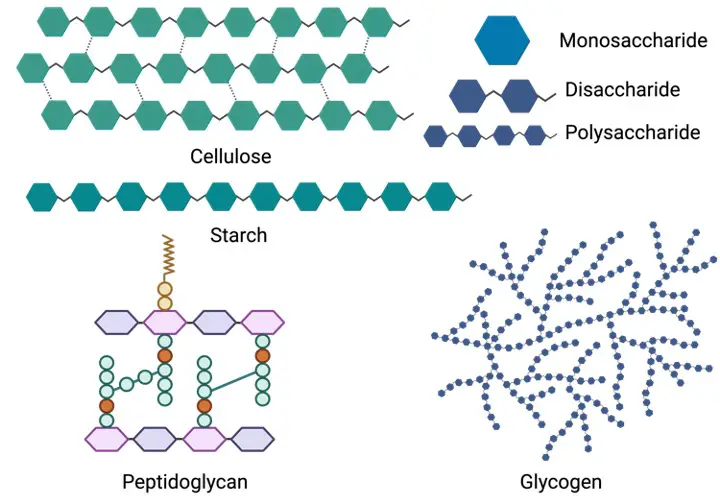
Carbohydrates’ structural (monomeric) unit is saccharide. Carbohydrates are classified as monosaccharide, disaccharide, oligosaccharide, or polysaccharide depending on the number of monomeric units.
Monosaccharides are simple sugar carbohydrates with only one sugar. Glucose, fructose, and galactose are examples. Carbohydrates of this kind are the most basic.
Disaccharides are carbohydrates that consist of two monosaccharide molecules joined together. Sucrose, maltose, and lactose are examples of sugars.
Oligosaccharides are polysaccharides made up of two to ten monosaccharide molecules. Raffinose, maltotriose, and maltotetraose are among examples. Polysaccharides are those having a large number of monosaccharide units.
Starch, cellulose, and glycogen are among examples. Homopolysaccharide (or homoglycan) refers to a polysaccharide made up of monosaccharide units of the same kind, whereas heteropolysaccharide refers to a polysaccharide made up of more than one type of monosaccharide (or heteroglycan).
iv. Lipids
Lipids are organic molecules that are soluble in nonpolar but not polar solvents (e.g., ether) (e.g water). Their main biological activities include energy storage, cell membrane structure, and cell signalling.

The lipid component of biological membranes contains a hydrophilic head, which can be a glycolipid, phospholipid, or sterol (such as cholesterol), and a hydrophobic tail.
v. Metabolites
Metabolites and natural products are examples of other biomolecules. Any chemical generated by metabolism or a metabolic process is referred to as a metabolite. Alcohols, amino acids, antioxidants, nucleotides, organic acids, vitamins, polyos, alkaloids, terpenoids, and other metabolites are examples. Biologically generated materials, bio-based materials, and physiological fluids are examples of natural goods.
Biomolecules Research
Biochemistry and molecular biology are two branches of biology that investigate biomolecules and their interactions. Molecular biology is the study of the structure and function of macromolecules that are necessary for life (and especially with their genetic role). The study of life at the molecular level, such as the chemical characteristics of DNA, is known as molecular biology.
The study of the structure and function of cellular components such as proteins, carbohydrates, lipids, nucleic acids, and other biomolecules, as well as their roles and transformations throughout life processes, is known as biochemistry.







