Table of Contents
Concentration Gradient Definition
Concentration originates from a French word, which means put at the center. However, gradient is a Latin word which means to work. Thus, concentration gradient is the change in the solute concentration within the solution. The solution comprises of two parts, the solvent in which the particles will dissolve and the solute which will break in the solution.
What is Concentration Gradient?
In biochemistry, concentration refers to the amount of solute present within a solution, whereas gradient pertains to the increase or decrease slowly in terms of distance. Thus, concentration gradient means there is a difference between the solute concentration in two solution.
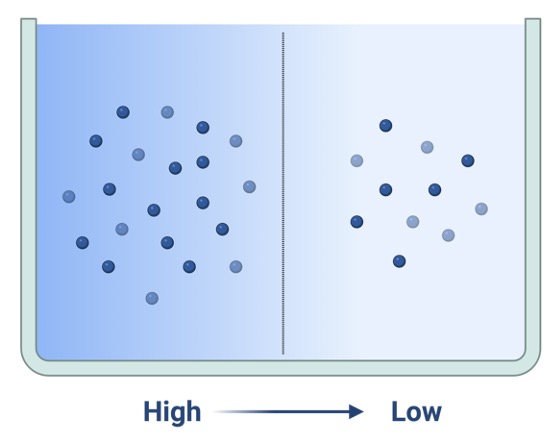
Concentration gradient refers to the improper division of particles in two solutions, which can be the intracellular and extracellular fluid. This solute difference causes them to drift from a highly dense environment to less dense environment, thus resulting in a balance between the solute concentration of two solutions.
Concentration Gradient and Biological Transport
It comprises of two transport types, the active and passive transport. The movement of particles through the concentration gradient where they move from a region of high concentration to a region of low concentration. This process does not require any energy.
Osmosis, facilitated diffusion, filtration and simple diffusion are the examples of passive transport. Active transport is the reverse of passive transport where the particles move against the concentration gradients, where particles from low concentration shifts to a high concentration region, and this process requires energy as molecules are moved to a dense area.
Concentration Gradient and Diffusion
A type of passive diffusion is the simple diffusion, which moves from an area of high concentration to an area of low concentration and is it’s a downhill movement transport proteins are not required and the driving force is concentration gradient, which when transport the molecules to the place, they possess no charge, thus the balance is maintained and the particle number as well is balanced in both the areas.

Facilitated diffusion, require driving force like the equilibrium and concentration gradient, however when there is no movement then only equilibrium can be achieved. Transport proteins are also required by facilitated diffusion. Only concentration gradient is not sufficient in passive transport. For example, through lipid bilayer, small lipid molecules and non-polar molecules can easily pass then the polar molecules.
Concentration Gradient and Osmosis
Water requires transport protein, in moving through the concentration gradient across the membrane. Osmosis and diffusion resemble each other as they move down and differ from each in particles. In osmosis, there is movement of solvent, whereas in diffusion it is the movement of solute.
In osmosis, the driving force is the osmotic gradient which moves the water from high concentration to an area of low concentration. But the movement across the cell membrane requires channel protein which spans the entire membrane making it a hydrophilic channel so that polar molecules like water can pass through.
As the membrane was previously a hydrophobic lipid bilayer, water is a polar molecule, thus it cannot pass through and requires a transport protein. However, no energy is required as the movement is downhill.
Concentration Gradient in Active Transport
Active transport is the reverse of passive transport where the particles move against the concentration gradients, where particles from low concentration shifts to a high concentration region, and this process requires energy as molecules are moved to a dense area.
Active transport is of two types primary and secondary. Secondary transport requires an electrical or chemical gradient whereas primary transport utilizes energy from ATP. Electrochemical gradient consist of electrochemical potential, where ions can pass in or out via the cell membrane.
As ions possess charge when they leave or enter their charge has an effect on the electric potential on the membrane. If the charge is imbalanced, then ions will move to balance the other side and regulate the balance.
Concentration Gradient Examples
i. Ion Gradient
An example of ion gradient is sodium/potassium gradient, which is important to cells. Neurons possess sodium/potassium pump, which is required for maintaining the resting membrane potential from 60-90mV. 3 sodium ions within the cell binds to protein pump and then gets phosphorylated by ATP resulting in a conformation, which will liberate three sodium ions outside the cell. then one potassium ion attaches to the pump and enters the cell.
The pump returns to its original place once the phosphate is liberated from ATP. Through this process, more negative ions are maintained within than the outside, which is required for action potential.
ii. Proton Gradient
In proton gradient or hydrogen gradient, the difference is formed due to proton concentration in the outer and inner biological membrane. Proton pump is responsible for transporting hydrogen ions through the membrane and forms a proton gradient. This gradient energy is vital as it reserves energy. This gradient is used for oxidative phosphorylation of cellular respiration.
The movement of protons from the mitochondrial matrix to the inter-membrane space is done by the proton pump, where are less protons inside than outside, resulting in a proton concentration gradient through inner mitochondria membrane.
iii. Respiratory Gas Concentration Gradient
The respiratory gases in animals are carbon dioxide and oxygen which when varies in concentration forms a concentration gradient in between the tissue liquid and the blood. Through the capillary beds, these gases move downwards.