Table of Contents
Nucleotide Definition
A nucleotide is the basic building block of nucleic acid. Examples of a nucleic acid include DNA and RNA. Nucleic acids are one of the types of biomolecules (other biomolecules are carbohydrates, proteins, and amino acids).
The major functions of nucleic acids are preservation, replication, and expression of hereditary information. They are also involved in the formation of nucleoside triphosphates. Nucleotides act as a secondary messenger in cell signaling in various cellular processes.
What is Nucleotide?
A single nucleotide is made up of a nitrogenous base, five-carbon sugar, and a phosphate group. The RNA molecule is composed of ribose sugar whereas DNA consists of deoxyribose sugar. The sugar molecules of each nucleotide monomer are connected by a phosphate group, thus the phosphate group and sugar moieties are considered as a backbone of nucleic acid.
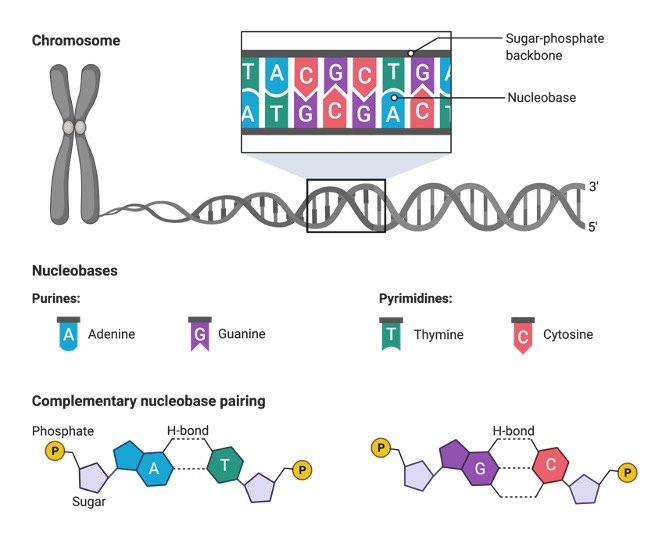
The opposite orientation of two strands of DNA allows complementary base pairing between nucleobase constituents. Nucleotides can occur in long chains as well as in cyclic forms. When the phosphate group is linked twice to the sugar moiety, it forms cyclic nucleotides. Nucleotides also act as energy carriers. The cell requires chemical energy for various cellular activities that are carried by the nucleotides.
Nucleotide vs Nucleoside
Sometimes both the terms nucleosides and nucleotides are confused with each other, but there is a difference between them. The nucleoside lacks the phosphate group. The nucleotide is formed when the nucleoside is bound to a phosphate group.
A nucleotide is also named nucleoside monophosphate (having a single phosphate group), nucleoside diphosphate (having two phosphate groups), or nucleoside triphosphate (consisting of three phosphate groups). A nucleoside is considered a ribonucleoside or deoxyribonucleoside depending upon the sugar component.
The nucleoside having ribose sugar is named ribonucleoside whereas the nucleoside having deoxyribose is considered as deoxyribonucleoside. A ribonucleoside may be guanosine, cytidine, uridine, adenosine, or 5-methyluridine based upon the nucleoside component.
Similarly, the deoyriboseribonucleooside may consist of deoxyadenosine, deoxyguanosine, deoxycytidine, deoxythymidine, or deoxyuridine. Based upon the nucleobase component, the nucleoside is of two types- purine and pyrimidine.
Classification of Nucleotide
Based on the structure of the nitrogenous base, the nucleotides are classified as purines and pyrimidines. Adenine and guanine bases are considered purines whereas pyrimidine bases include thymine and cytosine and uracil. The thymine base is replaced by uracil in RNA.
The nucleic acids are distinguished as DNA and RNA based upon the nucleobases. In DNA, thymine pairs with adenine whereas in RNA instead of thymine, uracil pair up with adenine. The nucleobases pairs such as G-C and A-T or A-U are referred to as base complements.
Types of Nucleotide
i. Nucleotides consisting a single phosphate group
• Adenosine monophosphate (AMP)
• Guanosine monophosphate (GMP)
• Cytidine monophosphate (CMP)
• Uridine monophosphate (UMP)
• Cyclic adenosine monophosphate (cAMP)
• Cyclic guanosine monophosphate (cGMP)
• Cyclic cytidine monophosphate (cCMP)
• Cyclic uridine monophosphate (cUMP)
• Deoxyadenosine monophosphate (dAMP)
• Deoxy guanosine monophosphate (dGMP)
• Deoxycytidine monophosphate (dCMP)
• Deoxythymidine monophosphate (dTMP)
ii. Nucleotides consisting a two phosphate group
• Adenosine diphosphate (ADP)
• Guanosine diphosphate (GDP)
• Cytidine diphosphate (CDP)
• Uridine diphosphate (UDP)
• Deoxyadenosine diphosphate (dADP)
• Deoxyguanosine diphosphate (dGDP)
• Deoxycytidine diphosphate (dCDP)
• Deoxythymidine diphosphate (dTDP)
iii. Nucleotides consisting a three phosphate group
• Adenosine triphosphate (ATP)
• Guanosine triphosphate (GTP)
• Cytidine triphosphate (CTP)
• Uridine triphosphate (UTP)
• Deoxyadenosine triphosphate (dATP)
• Deoxyguanosine triphosphate (dGTP)
• Deoxycytidine triphosphate (dCTP)
• Deoxythymidine triphosphate (dTTP)
De Novo Synthesis of Nucleotide
De Novo Synthesis pathway is used to produce nucleotides. The pathway completes in the liver of humans. The pyrimidine formation initiates with the formation of carbamoyl phosphate that includes a series of steps. In the first step, a biochemical reaction results in the formation of carbamoyl phosphate that involves bicarbonate, glutamine, ATP, and a water molecule.
The reaction is catalyzed by the enzyme carbamoyl aspartate transcarbamylase. Then, by intramolecular condensation, the ring closes and converts carbamoyl phosphate into dihydroorotate in presence of the enzyme dihydroorotase.
Later, the dihydroorotate is converted into orotate by dihydroorotate dehydrogenase. After the formation of the pyrimidine ring, 5-phospho-α-D-ribosyl-1-pyrophosphate (PRPP) reacts to orotate and form orotidine-5-monophosphate (OMP).
In the presence of the enzyme OMP decarboxylase, the OMP is decarboxylated and yield uridine monophosphate (UMP). Eventually, the dephosphorylation of ATPs and kinases produce UDP and UTP be a biosynthetic pathway. The purines are derived from nucleotide inosine monophosphate (IMP).
Amino acids glycine, glutamine, and aspartic acids are involved in the production of IMP from a pre-existing ribose phosphate. Then, the ribose5- phosphate reacts with ATP to form 5- phosphoribosyl-1-pyrophosphate (PRPP). PRPP is involved in both purine and pyrimidine synthesis as well as in NAD and NADP formation. Later, the IMP molecule is converted into adenosine monophosphate or guanosine monophosphate (GMP).
Nucleotide Degradation
The degradation pathways for purines, guanine, and adenine are as follows: The GMP is converted into guanosine by hydrolyzation. Later it is cleaved to free guanine.
• Guanine- xanthine- uric acid
• Adenosine- inosine- hypoxanthine- xanthine- uric acid
The purines are degraded and form uric acid, which is released from the liver in humans. The degraded purines may be reused and salvaged by enzyme APRT (adenine phosphoribosyltransferase) and HGPRT (hypoxanthine-guanine phosphoribosyltransferase).
The pyrimidines can be recycled by a salvage pathway. The salvage pathway for pyrimidines include:
• Enzyme uridine phosphorylase converts uracil into uridine. Later, uridine is converted into uridine monophosphate by enzyme nucleoside kinase.
• Thymine is converted into thymidine then converted into thymidine monophosphate. The reaction involves the enzymes thymidine phosphorylase and nucleoside kinase.
Nucleotide Function
The nucleotides are the precursors or monomeric units of nucleic acids. They are involved in cell signaling and metabolism as cofactors. Some common examples of cofactors are FAD, ATP, and NADP. The nucleoside triphosphates provide chemical energy for amino acid synthesis, protein synthesis, cell division, intercellular movements, etc.
Nucleotide Citations
- Nucleotide second messengers in bacterial decision making. Curr Opin Microbiol . 2020 Jun;55:34-39.
- Nucleotide Transport and Metabolism in Diatoms. Biomolecules . 2019 Nov 21;9(12):761.
- Nucleotide Sugars in Chemistry and Biology. Molecules . 2020 Dec 6;25(23):5755.
- Figures are created with BioRender.com
Share
Related Post

07 Postdoctoral Jobs at University of British Columbia, Vancouver, Canada
If you’re a PhD degree holder and seeking postdoctoral fellowships, University of British Columbia,

06 Fully Funded PhD Programs at University of Turku, Turku, Finland
If you’re a Masters degree holder and seeking Fully Funded PhD Programs, University of

17 Postdoctoral Jobs at University of Oxford, Oxford, England
If you’re a PhD degree holder and seeking postdoctoral fellowships, University of Oxford, Oxford,

03 Fully Funded PhD Programs at University of Essex, Colchester, England
If you’re a Masters degree holder and seeking Fully Funded PhD Programs, University of

10 Postdoctoral Jobs at University of California – Berkeley, California
If you’re a PhD degree holder and seeking postdoctoral fellowships, University of California –

19 Fully Funded PhD Programs at Technical University of Denmark, Denmark
If you’re a Masters degree holder and seeking Fully Funded PhD Programs, Technical University

