Table of Contents
Pyrimidine Definition
A heterocyclic aromatic molecule with a pyrimidine ring that is found in nucleic acids (such as DNA and RNA), proteins, starches, and other compounds.
What is Pyrimidine?
A nucleobase is a nitrogen-containing molecule that produces a nucleotide when linked to the pentose sugars, ribose or deoxyribose. The monomeric unit of nucleic acids such as DNA and RNA is the nucleotide.
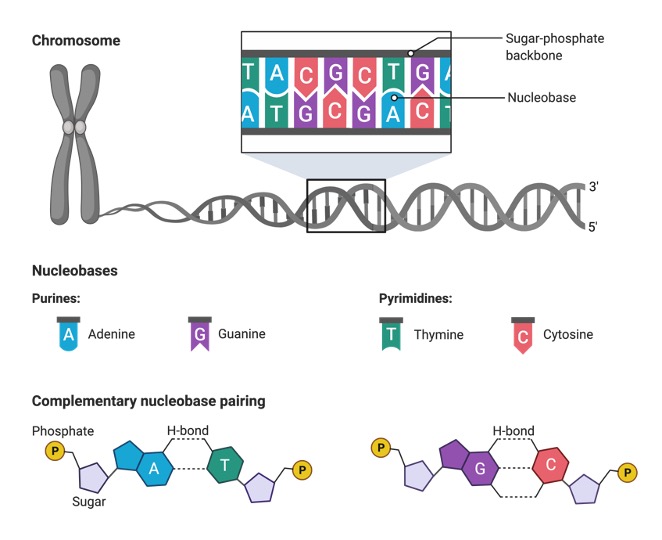
The nucleobases in two-stranded nucleic acids like DNA are paired. The complementary nucleobases are linked together via a hydrogen bond. Purines and pyrimidines are the two most common kinds of nucleobases.
Pyrimidine has the chemical formula C4H4N2 and is a heterocyclic aromatic organic molecule. It has a single ring (the pyrimidine ring) containing carbon and nitrogen atoms that alternate. Pyrimidine has a molar mass of 80.088 g/mol and a melting point of 20-22 °C.
Purines vs Pyrimidines
Purines and pyrimidines are both heterocyclic aromatic chemicals, but their chemical structures distinguish them from one another. Purines and pyrimidines both contain two carbon rings, however purines only have one.
A pyrimidine ring is joined to an imidazole ring in purine. There is only one pyrimidine ring in pyrimidine. Purine has four nitrogen atoms, while pyrimidine only has two. Pyrimidines feature cytosine, thymine, and uracil, while purines feature adenine and guanine.
Because they constitute the essential components of the genetic code, these five nitrogenous bases are referred to as main or canonical. To identify DNA from RNA molecules, the nucleobases that make up the nucleic acid are utilised. In DNA, thymine is complementary to adenine, but in RNA, uracil is complementary to adenine.
Thymine differs from uracil in that it has a methyl group, while uracil does not. Base complements are the pairings of the nucleobases C-G and A-T (or A-U in RNA).
Pyrimidine: Cytosine, Thymine, and Uracil
Pyrimidine nucleobases include cytosine, thymine, and uracil. Cytosine is differentiated from other pyrimidines by its heterocyclic aromatic ring, which has a keto group at position 2 and an amine group at position 4.
Its chemical formula is C4H5N3O. In DNA and RNA, cytosine establishes three hydrogen bonds with guanine. They become cytidine triphosphate (CTP) and deoxycytidine triphosphate (dCTP), which are nucleotides that structure RNA and DNA molecules, respectively, when phosphorylated with three orthophosphoric acid groups.
A cytidine triphosphate is a kind of nucleotide that is found in DNA and RNA. It may also serve as a cofactor for enzymes. It has the ability to transfer its phosphate in order to convert ADP to ATP.
C5H6N2O2 is the chemical formula for thymine. In its heterocyclic aromatic ring, it has two keto groups at positions 2 and 4, as well as a methyl group at position 5. Two hydrogen bonds connect the complimentary base thymine to the adenine base.
Unlike cytosine, which may be found in both DNA and RNA, thymine is only found in DNA since it is replaced by uracil in RNA. Thymine that has been bonded to a deoxyribose (a pentose sugar) (or thymidine) is referred to as deoxythymidine.Deoxythymidine forms deoxythymidine triphosphate (dTTP) when it is phosphorylated with three phosphoric acid groups.
dTTP is one of the nucleotide monomeric units that make up DNA. In terms of structure, uracil is identical to thymine except for the methyl group at position 5 in the heterocyclic aromatic ring found in thymine.
Its chemical formula is C4H4N2O2.Uracil pairs with adenine in complementary base pairing. In general, uracil is found in RNA rather than DNA. DNA has thymine, which pairs with adenine instead of uracil.
The conversion of cytosine to uracil by spontaneous deamination is one of the probable theories for why DNA includes thymine instead of uracil. When cytosine loses its amine group, it might transform into uracil. This cytosine deamination is a frequent occurrence. Nonetheless, the mistake is repaired by the DNA repair mechanisms that are built into the system.
However, if the damage is not healed, it may result in a point mutation. If uracil is present in the DNA, the repair mechanisms may not be able to tell the difference between the original uracil and the cytosine-turned-uracil, and therefore may not know which uracil to fix.
The inclusion of a methyl group in thymine (which is lacking in uracil) helps to prevent this from happening, maintaining the genetic code’s integrity and stability. Uridine is uracil that has been linked to a deoxyribose (a pentose sugar).
Uridine forms uridine triphosphate (UTP) when it is phosphorylated with three phosphoric acid groups. UTP is one of the nucleotide monomeric units that make up RNA.
Pyrimidine Metabolism
The ring is formed in pyrimidine biosynthesis by a sequence of processes that begin with the production of carbamoyl phosphate. First, bicarbonate, glutamine, ATP (for phosphorylation), and a water molecule are used in a biological process to generate carbamoyl phosphate. Carbamyl phosphate synthetase II, which is found in the cytosol, catalyses the process.
The enzyme aspartate transcarbamylase then converts the carbamoyl phosphate to carbamoyl aspartate. The ring then closes thanks to intramolecular condensation, with the enzyme dihydroorotase converting carbamoyl phosphate to dihydroorotate.
Finally, dihydroorotate is converted to orotate by dihydroorotate dehydrogenase (an integral membrane enzyme in the inner mitochondrial membrane). As a result, the bicarbonate ion (HCO3–) provides C2 of the pyrimidine ring, glutamine provides N3, and aspartate provides the remaining atoms in the rings.
After the pyrimidine ring develops, the ribose phosphate 5-phospho—D-ribosyl 1-pyrophosphate (PRPP) interacts with orotate to create orotidine-5-monophosphate (OMP). The enzyme OMP decarboxylase then decarboxylates OMP to produce uridine monophosphate (UMP).
Kinases and dephosphorylation of ATPs eventually create uridine diphosphate (UDP) and uridine triphosphate (UTP) farther along the biosynthetic route. By amination of UTP with the enzyme CTP synthetase, UTP can be transformed to cytidine triphosphate (CTP).
Purine biosynthesis varies from pyrimidine biosynthesis in that purines are formed first as a nucleotide, whereas pyrimidines are formed first as a free base. Pyrimidines are produced in a variety of organs in humans, including the spleen, thymus, and gastrointestinal system.
A salvage mechanism can be used to recycle degraded pyrimidines. After RNA and DNA breakdown, nucleobases are retrieved and reused. The following are the pyrimidine salvage pathways:
1. Deamination transforms cytosine into uracil. Uridine phosphorylase converts uracil to uridine by reacting with ribose-1-phosphate. Uridine is transformed to uridine monophosphate by the enzyme nucleoside kinase (UMP).
2. Thymine is transformed to thymidine by the enzyme thymidine phosphorylase interacting with deoxyribose-1-phosphate. The enzyme nucleoside kinase then converts thymidine to thymidine monophosphate.
One of the nucleobases, pyrimidines, is an essential structural component of nucleic acids. The genetic information required for all cellular activities and heredity is stored in nucleic acids such as DNA and RNA molecules.
Nucleobases are also essential components of some proteins and starches, in addition to nucleic acids. As a result, their roles include not just serving as structural components of DNA and RNA, but also regulating enzymes and cell signalling.
Pyrimidine Citations
- Fresh insights into the pyrimidine metabolism in the trypanosomatids. Parasit Vectors . 2018 Feb 8;11(1):87.
- Pyrimidine: A promising scaffold for optimization to develop the inhibitors of ABC transporters. Eur J Med Chem . 2020 Aug 15;200:112458.
- Disorders of purine and pyrimidine metabolism. Mol Genet Metab . Sep-Oct 2005;86(1-2):25-33.
- Figures are created with BioRender.com







