Table of Contents
What is Hemostasis?
Hemostasis is a natural mechanism that prevents blood loss after an injury. Hemo denotes blood, while stasis denotes a halt. Blood transforms from a liquid to a gelatinous condition during hemostasis.
Hemostasis Steps
Hemostasis is made up of three stages that happen in a quick succession:
- Vascular spasm, also known as vasoconstriction, is the temporary contraction of blood vessels.
- The platelet plug’s formation
- Blood clotting, also known as coagulation, strengthens the platelet plug by forming a fibrin mesh that works as a glue to keep the clot together. Tissue healing can occur once the blood flow has stopped.
i. Vasoconstriction
Healthy blood arteries are essential for preventing blood from clotting. Endothelial cells in healthy arteries prevent clotting by expressing a fibrinolytic heparin molecule and thrombomodulin, which inhibits platelet aggregation and uses nitrous oxide to terminate the coagulation cascade.
Endothelial cells stop secreting coagulation and aggregation inhibitors when they are injured, instead secreting von Willebrand Factor, which promotes platelet adhesion during the early development of the clot. During hemostasis, the vasoconstriction is a short reflexive contraction that produces a reduction in blood flow to that region.
ii. Platelet Plug Formation
Platelets produce a platelet plug very immediately when a blood artery is damaged. Coagulation begins within twenty seconds of an injury to the blood vessel’s epithelial wall being damaged. It takes around 60 seconds for the first fibrin strands to start interspersing across the incision.
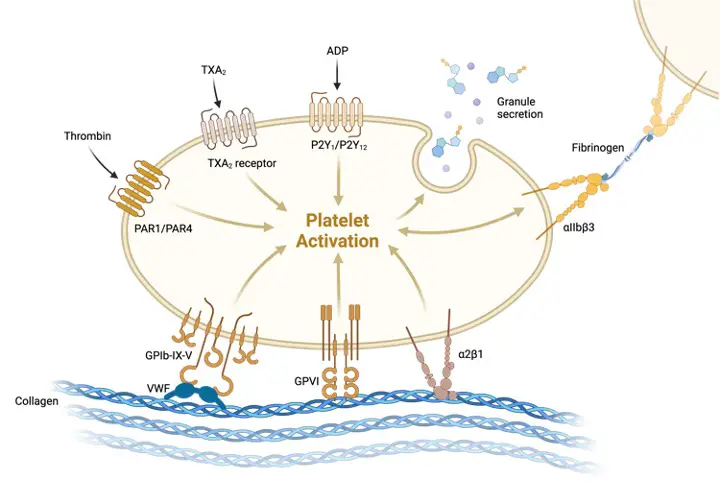
The fibrin totally forms the platelet block after a few minutes. Contrary to common perception, clotting of the skin is produced by platelets sticking to and being activated by collagen in the endothelium of blood vessels. The contents of the activated platelets’ granules, which contain a range of chemicals that encourage additional platelet activation and improve the hemostatic process, are subsequently released.
Thrombboxane causes platelets to expand, develop filaments, and start clumping together or aggregating when the lining of the blood artery breaches and the endothelial blood cells are injured, exposing subendothelial collagen proteins from the extracellular matrix. They stick to one other and the vessel’s walls due to Von Willebrand’s factor.
This process continues as additional platelets clump together and experience the same changes. A platelet plug forms as a result of this process, which closes the damaged region. A platelet plug can develop in a matter of seconds if the damage is minor.
iii. Coagulation Cascade
If the platelet plug fails to halt bleeding, hemostasis progresses to the third step, which is the development of a blood clot. Secretory granules are found in platelets. They degranulate when they attach to the protein in artery walls, releasing chemicals such as ADP adenosine diphosphate, serotonin, and thromboxane 2, which stimulate additional platelets.
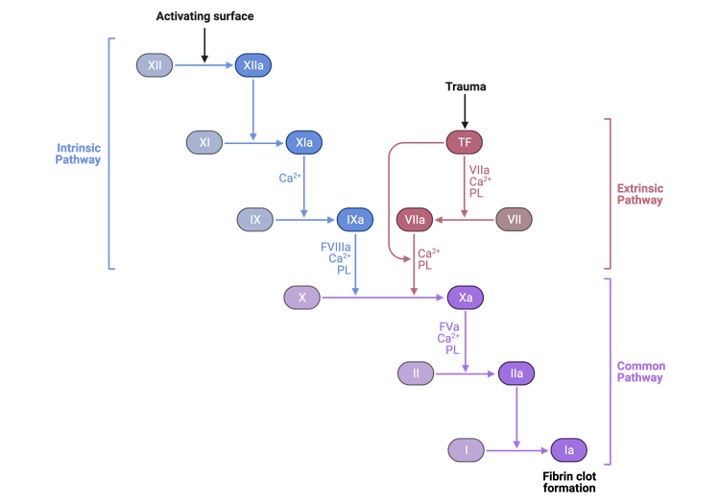
The blood first transforms from a liquid to a gel. At least 12 chemicals known as clotting factors or tissue factors are involved in a series of chemical events that result in the fibrin meshwork inside the blood. Each clotting factor has a distinct purpose. The key variables in the coagulation cascade’s result are prothrombin, thrombin, and fibrinogen.
The liver produces prothrombin and fibrinogens, which are proteins that are deposited in the blood. When blood arteries are injured, adjacent platelets are prompted to produce a chemical called prothrombin activator, which stimulates the conversion of prothrombin, a plasma protein, into thrombin, an enzyme. Calcium ions are required for this process.
This is where the calcium metabolism that we discussed before comes into play, which is present in both vitamin K2 and chitosan because both contain calcium ions. So this is when the entire hemostatic process comes to a halt.
Thrombin aids in the conversion of fibrinogen, a soluble plasma, into long insoluble fibres or threads of fibrin, a protein. Fibrin threads form an interlocking network of fibres and the structure for the clot as they loop around the platelet plug at the injured region of the blood artery.
The net of fibres catches and retains platelets, blood cells, and other substances close to the damage site, making the clot more effective. This transient fibrin clot forms in under a minute and restricts blood flow until platelets join. As a result, there is a link between calcium ions and calcium metabolism, which becomes engaged in the process and produces the first clot.
As a result, chitosan and Vitamin K2 interact with calcium ions in this way. Chitosan chitin and vitamin K2 have a lot in common. Chitin is a glucosamine-based polymer found in the shells and walls of crustaceans, fungus, and yeast. It is made up of calcium oxide and protein units and is the primary component of crustacean exoskeletons.
In crab shells, chitin accounts approximately 50-80% of organic molecules. Shellfish chitin is treated with alkali to produce chitosan, an amino polysaccharide. Chitin and chitosan are nonstarch polysaccharides that have the potential to be considered dietary fibre components.
Vitamin K3, on the other hand, has impacts on calcium ions and bone production, as stated above. As a result, there is a link between the body’s calcium metabolism and blood clotting that requires more investigation. The research has progressed.
Every day, experts come out with fresh research remedies for the same problem. Coagulation is now carried a step further by entering the coagulation pathways, which will be discussed in more detail in relation to calcium metabolism. Hemostatic arrest bleeding, which is the fundamental function of chitosan and vitamin K2, is a commonly utilised application for blood clotting.
In the therapy of wound healing, a functional chitosan-based hydrogel containing vitamin K2 can be used as a wound dressing and drug delivery system. Function active wound dressings are supposed to maintain a moist wound environment, protect against secondary infections, eliminate wound exudate, speed tissue regeneration, and increase wound healing efficiency.
Biodegradable, biocompatible, non-toxic, antibacterial, biologically adhesive, biological activity, and hemostatic properties make chitosan-based hydrogels excellent materials for aiding wound healing.
Chitosan-based hydrogels containing vitamin K2 have been shown to enhance wound healing at various phases of healing and to relieve variables that impede wound healing, such as excessive inflammatory response and persistent wound infection.
Because of the unique biological characteristics of chitosan-based hydrogel containing vitamin k2, it may be used as a wound dressing as well as a drug delivery system (DDS) to administer antibacterial agents, growth factors, stem cells, and other substances that can help speed wound healing.
By altering or combining with other polymers and containing various types of active ingredients, chitosan-based hydrogels can improve the efficiency of wound healing for a variety of wounds. Let’s take a deeper look at how chitosan-based hydrogels containing vitamin K2 may be used to improve wound healing and drug delivery systems.







