Table of Contents
Endoplasmic Reticulum Definition
Endoplasmic Reticulum is a membrane-bound organelle that takes the form of a labyrinthine network of flattened sacs or tubules linked to the nuclear membrane, flowing through the cytoplasm, and perhaps extending into the cell membrane.
What is Endoplasmic Reticulum?
“Organelles” literally translates to “tiny organs.” The cell, like the rest of the body, is made up of a number of organs that each have their own purpose. The definition of an organelle in some texts is rather specific. An organelle is a lipid bilayer-encased structure. Organelles include the nucleus, endoplasmic reticulum, Golgi apparatus, mitochondria, and chloroplast (plastid), although ribosomes and nucleosomes are not.

Lysosomes and vacuoles, on the other contrary, do not qualify as organelles because they are single-membrane cytoplasmic structures. Other sources, on the other hand, have fewer limitations. An organelle is a specialised component of a cell that serves a specific purpose. There are two sorts of organelles in this case: membrane-bound organelles (which include both double- and single-membraned cytoplasmic structures) and non-membrane-bound organelles.
Membrane-bound organelles also included the nucleus, endoplasmic reticulum, Golgi apparatus, mitochondria, plastids, lysosomes, and vacuoles. Non-membrane-bound organs tend to involve ribosomes, spliceosomes, vaults, proteasomes, DNA polymerase III holoenzyme, RNA polymerase II holoenzyme, photosystem I, ATP synthase, nucleosomes, centrioles, microtubule-organizing centres, cytoskeleton, flagellum, nucleolus, stress granules, as well as others.
Endoplasmic Reticulum Characteristics
The endoplasmic reticulum is one of a eukaryotic cell’s most visible organelles. The ER is a cytoplasmic organelle that initially appeared as a network of flattened sacs or tubules (called cisternae). The outer nuclear envelope is linked to the ER membranes. They could even penetrate the cell membrane.
The ER is one of the three components of the GERL system, which also includes the Golgi apparatus and lysosomes. It can be present in a variety of cell types. Red blood cells and spermatozoa, on the other hand, are devoid of it.
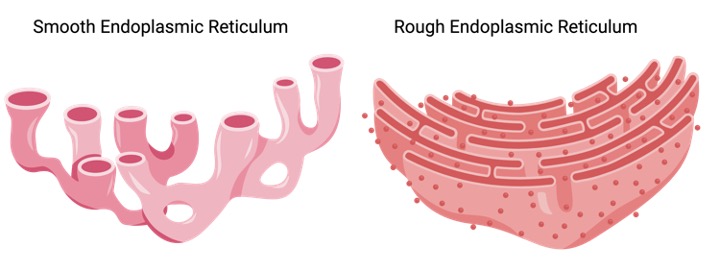
Types of Endoplasmic Reticulum
The rough endoplasmic reticulum (rER) and the smooth endoplasmic reticulum (sER) are the two types of ER. The rER’s outer surface is covered in ribosomes, giving it a rough look; hence its name. The sER, on the other hand, does not have ribosomes connected to its outer surface, making it smoother. Hepatocytes have a lot of rER, but mammalian liver and gonad cells have a lot of sER.
i. Rough Endoplasmic Reticulum
A translocon is a protein complex involved in the translocation of nascent polypeptides from the cytosol to the inner surface of the rER membranes. The translocon acts as a docking point for ribosomes in the endoplasmic reticulum. The bound ribosomes, on the other hand, are only temporarily attached to the ER.
They have complete freedom to come and go as they choose. When a signal peptide is synthesised (i.e. by protein translation at the ribosome) and then recognised by a signal recognition particle, it attaches to the endoplasmic reticulum (via the translocon).
ii. Smooth Endoplasmic Reticulum
The sER is a network of tubules and vesicles that branch to increase surface area for the action or storage of key enzymes. It is connected to the nuclear envelope. It contains the gluconeogenesis enzyme Glucose-6-phosphatase (which converts glucose-6-phosphate to glucose). In muscle cells, where calcium ions are stored, a specialised form of sER is found. The sarcoplasmic reticulum is what it’s called.
Hepatocytes have a lot of sER, which helps them process and detoxify lipophilic drugs. Sebaceous gland cells and gonad cells are two other specialised cells that have a lot of sER (e.g. testes and ovaries).
Endoplasmic Reticulum Function
Because rER has ribosomes attached to its surface, it is involved in protein synthesis and folding, as well as protein shuttling to the Golgi apparatus, where the nascent protein is matured. It produces serum proteins (such as albumin) and hormones (such as insulin) as well as other compounds (such as milk) in the liver and glands.

The rER also plays a role in the production of lysosomal enzymes (in which a marker, mannose-6-phosphate, is later added in the Golgi apparatus). It’s also where some of the membrane’s integral proteins are made. N-linked glycosylation is also present (O-glycosylation occurs in the Golgi).
The surface of the sER, on the other hand, is devoid of ribosomes. Its activities include lipid synthesis, carbohydrate metabolism (i.e., the conversion of glucose-6-phosphate to glucose during glucogenesis via the sER enzyme glucose-6-phosphatase), drug detoxification, and receptor attachment to cell membrane proteins. It also plays a role in intracellular transport, such as transporting rER products to other cell sections such as the Golgi apparatus.
Biological Reactions in Endoplasmic Reticulum
Protein synthesis is the method of constructing protein molecules. The key processes in biological systems are amino acid synthesis, transcription, and translation. Transcription is a nuclear event in which an mRNA template is transcribed from DNA to give a template for translation.
The mRNA template encodes the sequence of the protein in the form of a trinucleotide code. Ribosomes are the location of translation, which is a cytoplasmic process. There, tRNAs add the amino acids, which are subsequently joined together in the order indicated in the mRNA transcript.
Maturation mechanisms such as proteolysis, post-translational modification, and protein folding occur after these events. A signal peptide is created early in the translation process (i.e. by protein translation at the ribosome).
The signal indicates that the protein will be processed further in the ER. The ribosome that is translating the protein attaches to the endoplasmic reticulum through the translocon when this signal is detected by a signal recognition particle. The ribosome then returns to the protein translation process.
As the mRNA transcript is translated by the docked ribosome, the chain continues to expand. The chain finally makes its way into the ER through the translocon, which bridges the membranes of the ER.
In the ER lumen, a signal peptidase degrades the signal peptide. Chaperone proteins (such as ERp29, protein disulfide isomerase, BiP/Grp78, calnexin, and others) fold the nascent protein in the ER. The correctly folded protein is subsequently packaged into a transport vesicle and transported to the Golgi apparatus, where it is matured for transport through the cytoskeleton to other cytoplasmic organelles such as lysosomes and peroxisomes, or for secretion out of the cell.
As the mRNA transcript is translated by the docked ribosome, the chain continues to expand. Through the translocon, which bridges the ER membranes, the chain finally makes its way into the ER.

A signal peptidase enzyme in the ER lumen removes the signal peptide. Chaperone proteins (such as ERp29, protein disulfide isomerase, BiP/Grp78, calnexin, and others) fold nascent proteins in the ER.
The correctly folded protein is subsequently loaded onto a transport vesicle and transported to the Golgi apparatus, where it is matured for transport through the cytoskeleton to other cytoplasmic organelles such as lysosomes and peroxisomes, or for secretion out of the cell.
The enzyme UGGT is responsible for the first response of glycosylation (UDP-glucose: glycoprotein glucosyltransferase). The heat shock protein regulate protein attach to the misfolded protein’s hydrophobic residues, preventing it from transiting.
If the protein continues to misfold, it will be degraded to prevent it from accumulating with other misfolded proteins. The ERAD chaperone transports the misfolded protein to the cytosol for destruction by cytosolic proteasomes via endoplasmic reticulum-association degradation (ERAD) (via the ubiquitin-proteasome pathway). If these procedures fail to restore the cell’s normal function within a specific amount of time, the cell’s next response is apoptosis.
Lipid production takes place mostly in the sER, notably at membrane contact sites (MCS). MCS are regions in which ER membranes come into intimate contact with other cytoplasmic organelles, including as the Golgi, mitochondria, lysosomes, peroxisomes, endosomes, chloroplasts, and plasma membrane, allowing chemicals to be transferred. The production of phospholipids is enabled via contact areas between the ER and mitochondria.
Endoplasmic Reticulum Citations
- The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci . 2016 Jan;73(1):79-94.
- Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature . 2016 Jan 21;529(7586):326-35.
- The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol . 2015;10:173-94.
- Figures are created with BioRender.com







