Phosphodiester Bond Definition
A reaction of two hydroxyl groups in phosphoric acid with a hydroxyl group on other molecules forming ester bonds results in the formation of phosphodiester bonds.
The backbone of the strands of nucleic acid is made of phosphodiester bonds. The bond formation takes place by reacting the hydroxyl group of phosphoric acid with a hydroxyl group on other molecules forming ester bonds. The phosphodiester bond is formed when phosphate forms two ester bonds.
What is Phosphodiester Bond?
The condensation reaction between phosphate groups and hydroxyl groups of two sugar molecules results in the formation of a phosphodiester bond. The hydroxyl group is formed by the bonding of one oxygen group and one hydrogen group. It can be written as –OH, or –HO. Here, the – sign indicates the carbon chain. Phosphate groups are molecules having four oxygen atoms covalently bonded with one phosphorus atom.it is also known as a phosphodiester bond.
Are Phosphodiester Bonds Covalent?
Phosphodiester bonds are formed by sharing of electrons in between their atoms and thus it is defined as phosphodiester bonds. The enzyme phosphodiesterase can break the phosphodiester bonds. They are abbreviated as PDE. Based upon the amino acid sequences, substrate specificity, regularity, pharmacological properties of these enzymes, PDE is categorized into 12 subfamilies.
Phosphodiester Bond Formation
The reaction between two hydroxyl groups of two sugar groups and a phosphate group results in the formation of phosphodiester bonds. The combination of the diester bond in the phosphoric acid results in the formation of oligonucleotide polymers between sugar molecules present in the DNA and RNA molecule.
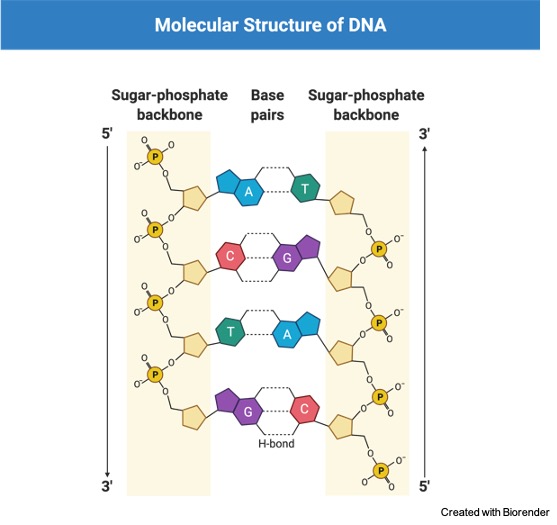
DNA and RNA are a type of nucleic acids. The DNA has 3’ carbon linked with the 5’ carbon via the phosphodiester bonds. The bond holds the sugar-phosphate components of the DNA molecule together therefore it acts as the backbone of nucleotides.
Permeability: Definition, Types, & Examples
Nucleotides are the monomeric unit of nucleic acids. A nucleotide is formed with a nitrogenous base, a pentose sugar, and a phosphate molecule. The DNA consists of nitrogenous bases named adenine, thymine, guanine, and cytosine. In the RNA the thymine is replaced by uracil.
The polymerization of nucleotides forms nucleic acids by the formation of ester bonds. A linkage has formed after the elimination of a water molecule.
The condensation process is used for the polymerization of RNA and DNA. In this process, the condensation of the strands of DNA or RNA occurs with the nucleotide triphosphate. As the result of the condensation reaction, a single nucleic acid strand is formed.
Phosphodiester Bond of DNA & RNA
The nucleic acids are the polymeric diesters of phosphoric acids. It is the biomolecules that store and transfer biological information among generations. The cleavage of phosphodiester linkage by phosphodiesterase enzyme is a very important biological process.
There are many diversified enzymes used to cleave the diester linkage of 5’- of one nucleotide to the 3’O of another nucleotide. The phosphodiester bond of DNA hydrolyzes either to the 5’- phosphate group or 3’ phosphate group whereas the RNA undergoes transesterification to a 2’- or 3’- cyclic phosphate. The process results in the hydroxylation of 2’- or 3’- phosphate groups.
Based on various research, it is assumed that the phosphodiester linkages are extremely stable. The RNA molecules also have extremely labile phosphodiester linkages due to the presence of hydroxyl functions. The RNA cleaves the RNA phosphodiester bonds by itself, this arrangement is called ribozyme.
Phosphodiester Bond Mechanism
The factors or parameters affecting the formation of phosphodiester bonds include the profiles of pH rates, relationships of linear free energies, and experimental approaches. The formation of phosphodiester bond includes two most common mechanisms, named as kinetic heavy atom isotope effect, and kinetic solvent isotope effect.
Both enzymatic and non-enzymatic reactions use kinetic heavy atom isotope atom whereas the KSIE or kinetic solvent isotope method is used to distinguish between the alternative mechanisms.
Biological Importance of Phosphodiester Bond
Phosphodiester bonds act as the backbones for nucleic acid strands. Therefore they play a significant role in the formation and stability of DNA and RNA that carry the hereditary information of the cell. DNA is the genetic material is most of the organisms however some virus consists RNA as genetic material.
In humans, DNA is the genetic material that controls all cellular functions. RNA also plays an important role in protein synthesis. The structure of DNA and RNA is maintained by phosphodiester bonds thus it plays an important role in the formation of nucleic acids.
Phosphodiester Bond Citations
- The human 2′-5’oligoadenylate synthetase family: unique interferon-inducible enzymes catalyzing 2′-5′ instead of 3′-5′ phosphodiester bond formation. Biochimie . Jun-Jul 2007;89(6-7):779-88.
- DNA ligase: structure, mechanism, and function. Science . 1974 Nov 29;186(4166):790-7.