Table of Contents
Phospholipids Definition
- Phosphate-containing lipids have one or more phosphate groups.
- A lipid that is made up of glycerol, two fatty acids, and a phosphate group.
Phospholipids are a lipid subclass. Fatty acids, sphingolipids, sterol lipids, and prenol lipids are some of the other primary kinds of lipids. Lipids are organic molecules that are soluble in nonpolar but not polar solvents (e.g., ether and water, respectively).
Phospholipid is made up of two words: phosphor–, which comes from the element phosphorus, and –lipid, which comes from the Greek word lipos, which means fat.
What are Phospholipids?
Phospholipids are a kind of lipid that is found in many biological membranes, including the lipid bilayer of cells. This is due to the fact that phospholipids are amphipathic molecules, with the hydrophilic ‘head’ and the lipophilic ‘tail’ being hydrophobic.
Two long fatty acid chains generally make up the ‘tail.’ A glycerol component and a negatively charged phosphate group make up the ‘head.’ The phosphate group is linked to one of the glycerol backbone’s three carbons, while the other two are bonded to two fatty acid chains (mostly a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2).
Phosphate can also be attached to hydrogen, choline, serine, ethanolamine, inositol, and other molecules. Phosphatidic acid, phosphatidylcholine, phosphatidylserine, phosphatidylethanolamine, phosphatidylinositol, and other phospholipids have different hydrophilic components.
Each of these phospholipids has a distinct biosynthetic route. The most basic of these phospholipids is phosphatidic acid, which acts as a precursor to a variety of phospholipids. Phospholipid biosynthesis generally begins at Gro3P.
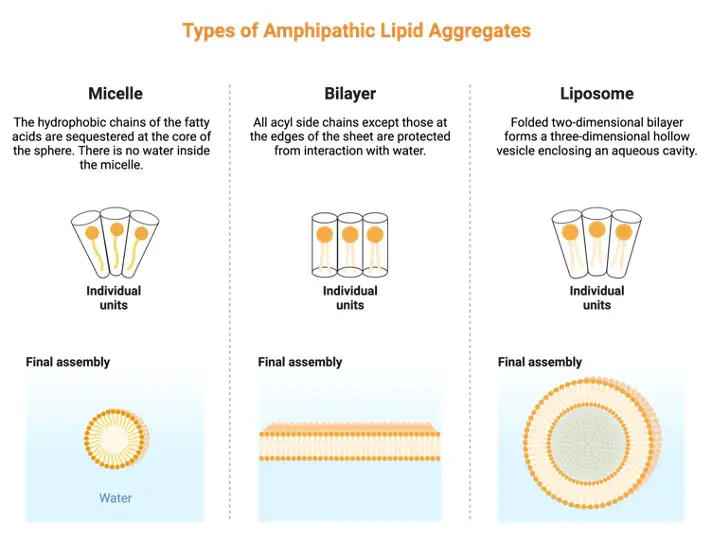
The amphipathic nature of the phospholipid tails prevents them from interacting with water. Water, on the other hand, may interact with the phospholipid heads. When phospholipids are put in water or an aqueous solution, their tails prefer to orient towards one other, causing them to aggregate.
As a result, the ‘heads’ are angled toward water or an aqueous solution. As a result, the phospholipid component of biological membranes permits the “lipid bilayer” that distinguishes them. The phospholipid tails align internally, whereas the phospholipid heads face outward on both sides. The presence of sterols in biological membranes prevents phospholipids from packing together (another group of lipids).
Biological Importance of Phospholipids
Phospholipids are amphipathic molecules with a hydrophilic ‘head’ and a lipophilic ‘tail’ that is hydrophobic. This is required for the development of biological membranes’ lipid bilayers. Most biological membranes, such as cell membranes, contain phospholipids as a key structural component.
The function of the cell membrane is dependent on phospholipids. Because they are amphipathic, their presence forms an effective barrier that prevents all molecules from entering. All molecules would be able to enter the cell, but not all of them would. Only tiny molecules (such as oxygen and carbon dioxide) and non-polar molecules are allowed to pass through the lipid bilayer.
Other molecules, particularly polar compounds, would require carriers or transport mechanisms to pass through the cell membrane. Phospholipids are also important in cell signalling and metabolism.
Phosphatidylinositol (4,5)-bisphosphate, for example, is broken down into inositol triphosphate and diacylglycerol (by the action of the enzyme phospholipase C). They work together as second messenger molecules in biological cells to help in signal transduction.
Phospholipids Citations
- Nonenzymatic Reactions above Phospholipid Surfaces of Biological Membranes: Reactivity of Phospholipids and Their Oxidation Derivatives. Oxid Med Cell Longev . 2015;2015:319505.
- Cell biology of cardiac mitochondrial phospholipids. Biochem Cell Biol . 2004 Feb;82(1):99-112.
- Phospholipids in foods: prooxidants or antioxidants? J Sci Food Agric . 2016 Jan 15;96(1):18-31.
- Figures are created with BioRender.com







