Table of Contents
What are Base Pairs?
Base Pairs are the sets of hydrogen-linked nucleobases that structure the nucleic acids DNA and RNA are mentioned as base pairs. Dr. Francis Crick and Dr. James Watson, who are most known for finding the helical, or “twist around,” structure of DNA, were the first to characterize them (1953).
DNA was also discovered to be the source of transmitting material during cell division at this period. Watson and Crick anticipated that the two strands of DNA can interweave using rule-abiding hydrogen bonds in their model.
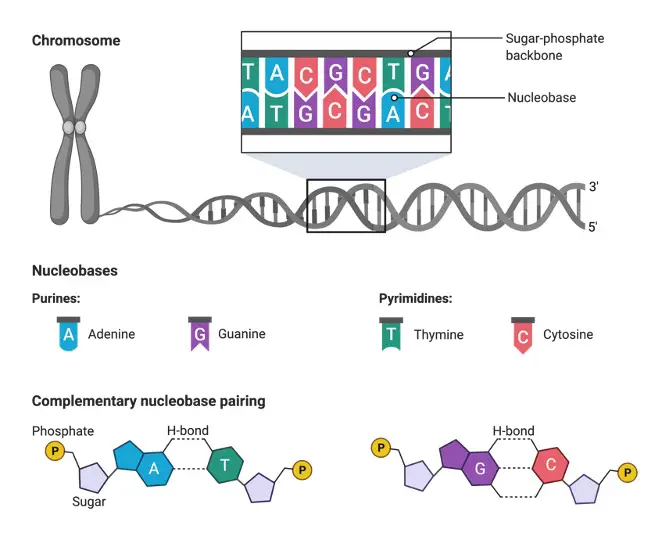
Adenine, thymine, guanine, and cytosine are the four kinds of nucleobases that make up our DNA. Nucleotides are the biological “building blocks” that allow life to exist and thrive. Each of which has a nitrogenous base, a sugar (deoxyribose in DNA), and a phosphate group.
The hydrophilic exterior “backbone” of the DNA helix is formed by the sugar and phosphate groups, whereas the nitrogenous bases point toward the nonpolar, hydrophobic centre. The purine class includes adenine and guanine, whereas the pyrimidine class includes cytosine and thymine. These bases are held together by a set of special base pair rules, which are explained further down.
Purine, Pyrimidine and Base Pairs
Purine bases, which are made up of adenine and guanine, are “PURe As Gold.” Cytosine + Thymine = Pyrimidine bases
Watson and Crick base pairs adhere to a particular hydrogen bonding rule. One purine nucleic base connects with one pyrimidine nucleic base in complementary pairing. Only two hydrogen bonds are formed when adenine and thymine are combined in DNA. To put it another way, this pair generates a strong double bond that keeps the dimers together.
On the other hand, cytosine and guanine create three hydrogen bonds, allowing for a shorter and more rigid connection. These nitrogenous bases are all planar molecules, which means they are relatively flat and stiff. This, of course, has the advantage of making DNA a more durable structure, which is critical because it includes our whole genetic code, which we must safeguard and maintain.
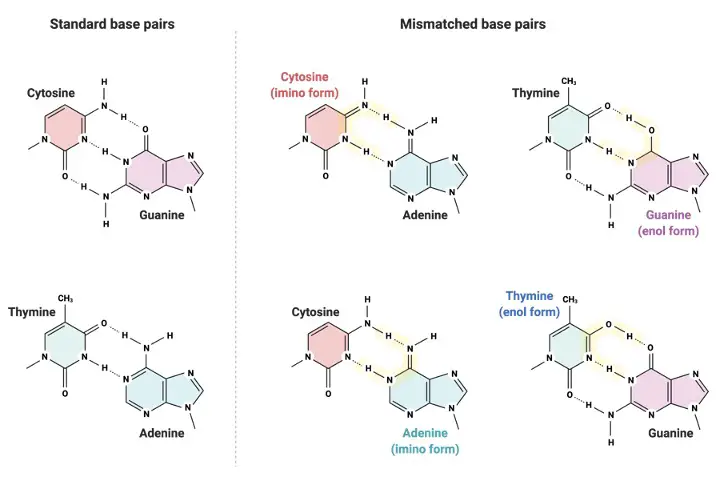
Another feature of base pairs is that the dimers that form have the same size and take up the same amount of three-dimensional space. This enables DNA to adopt a “steric fit” that ensures a consistent helical shape throughout. While the ratios of C+G: A+T nucleotides vary from creature to organism, the quantity of adenine and thymine in the organism will always match, as will the ratio of cytosine to guanine (known as “Chargaff’s rule”).
Watson-Crick Base Pair Hydrogen Bonds
1. Adenine + Thymine = Oxygen/Hydrogen and Nitrogen/Hydrogen create two hydrogen bonds.
2. Cytosine + Guanine = Oxygen/Hydrogen and Nitrogen/Hydrogen create three hydrogen bonds.
3. Keep in mind that hydrogen bond donors only have H atoms linked to electronegative atoms like Nitrogen or Oxygen. Electronegative atoms having at least one pair of lone electrons are hydrogen bond acceptors.
Base Pairs in RNA
Although RNA follows the Watson-Crick base pair rules, there are significant structural variations to be aware of. Although there are cases of single-stranded DNA and double-stranded RNA (i.e. RNAi), RNA is generally thought of as single-stranded and DNA as double-stranded.
Other distinctions include the fact that RNA has ribose as its sugar base and employs uracil rather than thymine as a nucleotide. Because uracil and thymine have comparable structural similarities, they may both base pair with adenine in the same way.
RNA, like DNA, is considerably shorter and comes in a variety of forms, including mRNA, which is the amazing molecule that gets translated into every protein in our cells and bodies.
Base Pairs Citations
- Products of Oxidative Guanine Damage Form Base Pairs with Guanine. Int J Mol Sci . 2020 Oct 15;21(20):7645.
- Metal-Modified Nucleic Acids: Metal-Mediated Base Pairs, Triples, and Tetrads. Angew Chem Int Ed Engl . 2020 Jan 20;59(4):1397-1406.
- Unnatural Base Pairs for Synthetic Biology. Chem Pharm Bull (Tokyo) . 2018;66(2):132-138.
- Figures are created with BioRender.com