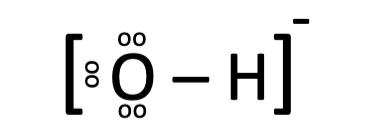What are Polyatomic Ions?
o An ion is defined as a chemical atom, molecule or particle that has a positive or a negative charge.
o The positive charge is present due to a greater number of protons than electrons in atomic structure. These positively charged ions are named as cation.
o On the other hand, if the number of protons is less than the electrons the ion then this type of ion is named as anions.
o When a compound is formed between positive and negative charge ions there is a strong electrostatic force between these ions then it is known as ionic bond and the compound denoted as ionic compound.
Types of Ions
Ions are of two types as mentioned below;
I. Monoatomic Ions
If an ion is made up of only one type of atom, it is called monoatomic ion.
For Example: Na+, Cl–, K+
II. Polyatomic Ions
o If ion is made up of two or more atoms, then this type of ion is called as polyatomic ion or a molecular ion.
o Example for the same include: NH4+ CO3-2 etc.
o Polyatomic ions are covalently bonded atoms. These behave as a single unit.
o It has net charge is not zero.
o The word Poly means “many” in Greek language and in old literature polyatomic ions are referred to as Radical. These take part in numerous chemical reactions like acid-base, precipitation, and displacement reaction.
o A polyatomic ion is referred to as the conjugate acid or base of a neutral molecule.
o For example, CaCO3 the anion carbonate (CO3-2) is the conjugate base of a weak acid (Carbonic acid) Compound made from these ions are water soluble and also conduct electricity and dissociate in a given solutions alike monoatomic ions.
How to Show a Charge on a Given Ion?
1. Firstly, write the chemical formula of the given ion.
2. Write the symbol of the charge on a given ion in superscript with the magnitude of the charge in superscript.
3. Lastly, the magnitude of the given charge can be omitted if charge is +1 or -1.
For instance Na+1 can be written as Na+ only.
Polyatomic Ions Examples
i. Hydroxide Ion
It comprises of one oxygen atom and one hydrogen atom carrying a charge of -1.
o The distinct line shows the sharing of 2 electron means the presence of covalent bond between H and O.
o The dots around O displays lone pairs of electrons.
o Total protons = protons in H +protons in O That is 1+8=9protons
o As Hydroxide ion has net -1 charge as it has one electron more than protons thus it means, nine protons and ten electrons.
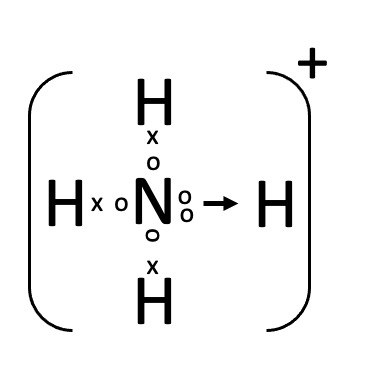
ii. Ammonium Ion
o Maximum polyatomic ions comprises of a negatively charged anions and oxygen but Ammonium is a type of ion that does not contain oxygen.
o As shown in above figure, ammonia (NH3) has a lone pair of electrons which it donates to proton and thus forms a coordinate bond hence called an Ammonium ion.
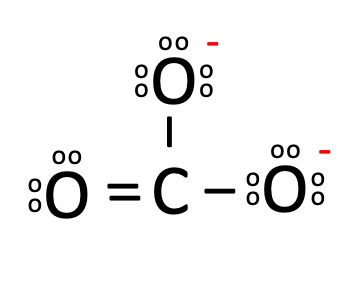
iii. Carbonate Ion
o As shown in below figure, carbon has four electrons in its valence shell and oxygen has six electrons.
o Thus, after forming three covalent bonds with oxygen the carbon is left with only 6 valence electrons so, there is movement of one pair of electrons from oxygen towards carbon atom.
o Therefore, forming a double bond, by which both carbon and oxygen attain octet condition.
Polyatomic Ions Types
o Polyatomic ions are for example; H3O+, NH4+
o Polyatomic anions occur in “families”. All have a central element and the same charge. Three types of variation are seen as mentioned below;
o Different members have different numbers of oxygen
o Each member can thus attach with hydrogen ions to reduce their negative charge
o In some members oxygen can also be replaced by Sulphur.
How Ions are Formed?
Given below are few methods by which ions are formed;
i. Physical Ionization
Spontaneous collision between liquid or gaseous molecules causes one of the free electrons to release off an atom or molecule. This way produce cation or anions.
ii. Chemical Interaction
o When a salt is dissolved in water or any other suitable solvent, the atoms thus undergo dissociation to form free ions.
o For instance, NaCl (common salt) when dissolved in water dissociates into sodium cation and chloride anions.
NaCl → Na+ + Cl–
iii. Passing of Electric Current
Ions can also be formed when an electric current is passed through solution of salts.
Polyatomic Ions Chart
| Carbon | Nitrogen | Sulphur | Chlorine | Phosphorus |
| CO32 carbonate HCO3 hydrogen carbonate (bicarbonate) |
NO3 nitrate
NO2 nitrite |
SO42- sulphate
SO32- sulphite HSO4– hydrogen sulphate (bisulphate) |
ClO4– perchlorate
ClO3– chlorate ClO2– chlorite ClO hypochlorite |
PO43 phosphate
HPO42 hydrogen phosphate H2PO4 dihydrogen phosphate |
Polyatomic Ions Chart
When these polyatomic ions form compound, remember two main things as mentioned below:
1. If there are more than one polyatomic ion of similar kind, we place a parenthesis around the ion’s formula and afterwards use a subscript to show how many ions are present in the given compound.
2. The overall charge for the ionic compound should be neutral, which means sum of positive and negative charge on ions should be equal to zero. For instance the formation of calcium hydroxide compound: Calcium (Ca) has 2+ charge. Hydroxide (OH-) carries 1- charge. While lettering formula of compound we use parentheses around OH followed by a subscript of 2. Therefore, the formula becomes Ca (OH) 2.
You may like to read;
Digestive System Disorder: Types, Definition, and Treatment
Human Respiratory System: Definition, Mechanism, and Facts
Blood Group: ABO, Types, Uses, and Facts
Lymph: Definition, Function, and Facts
Krebs Cycle: Definition, Diagram, Steps, and Mechanism
Polyatomic Ions Citations
- Comparison of primary monoatomic with primary polyatomic ions for the characterisation of polyesters with static secondary ion mass spectrometry. Rapid Commun Mass Spectrom . 2005;19(4):552-60.
- Polyatomic ions from a high current ion implanter driven by a liquid metal ion source. Rev Sci Instrum . 2017 Dec;88(12):123302.