Table of Contents
Maltose Definition
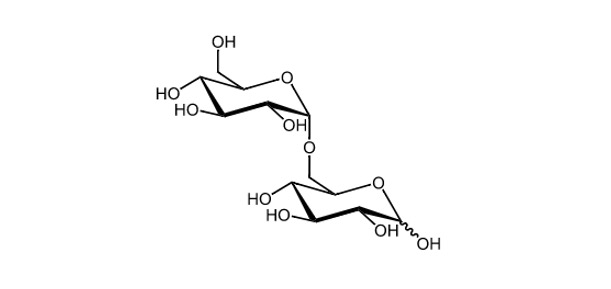
Maltose is a structural unit of glycogen and starch; a reducing disaccharide, which is produced when two glucose monomers link together via α(1→4) glycosidic bond.
What is Maltose?
Maltose is one of the most prevalent disaccharide carbohydrates; sucrose and lactose are two additional examples. Carbohydrates are a large group of biomolecules that are categorised according to their saccharide content. A disaccharide is a carbohydrate that consists of two monosaccharides joined by a glycosidic bond (glycosidic linkage).
Maltose is a crystalline white substance. The molar mass of this substance is 342.30 gmol1. (i.e. 102 °C) is its melting point. It is water soluble. Maltose has the same general formula as sucrose and lactose: C12H22O11.
Maltose, on the other hand, is a disaccharide that consists of two glucose molecules. The glucose components are joined by α-1→4 glycosidic bond, which produces a covalent connection between the -anomeric form of Carbon-1 (C-1) on one glucose and the hydroxyl oxygen atom on C-4 on the other.
The resultant molecule is cellobiose when the glycosidic link is β-(1→4). Another isomer of maltose is isomaltose. They’re both made up of two glucose units linked together by a glycosidic bond. Despite this, isomaltose varies from maltose due to the glycosidic bond: maltose has α-1→4 glycosidic link, whereas isomaltose has a α-1→6 glycosidic bond.
Discovery of Maltose
Maltose was discovered by Augustin-Pierre Dubrunfaut (1797–1881), a French chemist. In addition, he is credited with being the first to discover fructose. His discovery of maltose, however, was not widely recognised until 1872, when chemist Cornelius O’Sullivan (1841–1907) verified it. The term “maltose” is derived from the words malt (a germinated grain used in brewing, distilling, and other processes) and the suffix -ose, which denotes that it is a sugar.
Maltose vs Lactose
The three most prevalent dietary disaccharides are maltose (malt sugar), lactose (milk sugar), and sucrose (table sugar). The three disaccharides, as previously stated, have the identical chemical formula: C12H22O11. Glucose is present in all three. Maltose is a substance made composed of two glucose units.
However, just one glucose unit interacts with another monosaccharide in lactose and sucrose, resulting in galactose and fructose, respectively. The α-(1,4) glycosidic link connects the two sugars in maltose, i.e. Carbon-1 and Carbon-4. Carbon-1 of galactose and Carbon-4 of glucose form a β-(1,4) glycosidic link in lactose. The carbon-1 of glucose and the carbon-2 of fructose create a link in sucrose.
Sucrose is a non-reducing sugar, while maltose and lactose are reducing sugars. Because one of the monosaccharide components may include a free aldehyde group, maltose and lactose are reducing sugars. The glycosidic link develops between the reducing ends of the two monosaccharide components in the case of sucrose. As a result, sucrose could no longer combine with other saccharide units.
Dietary maltose is not found in most foods, although it can be produced when starch is digested. Lactose, on the other hand, is commonly found in milk and dairy products, whereas sucrose is commonly found in foods sweetened with sugar derived from sugar cane and sugar beet.
Specific digestive enzymes, such as maltase, lactase, and sucrase, help in the digestion of these sugars. These enzymes are found on the outside of the epithelial cells that lining the small intestine in humans.
Maltase aids in the digestion of maltose, lactase (β-galactosidase in bacteria), and sucrase (sucrose). The bond between the two monosaccharide components is cleaved by these enzymes. Maltose is a sugar that is sweeter than lactose. Sucrose, on the other hand, is the sweetest of the three.
Importance of Maltose
Maltose is made up of two glucose units linked together by a -14 glycosidic bond. When these two monosaccharides come together, water is released. More complex carbohydrates are formed when numerous maltose molecules are joined together, such as starch in plants and glycogen in mammals. Dehydration synthesis is a process in which the creation of glycosidic bonds occurs simultaneously with the release of water.
Saccharification is the process of breaking down complex carbohydrates into simpler ones. Dehydration synthesis is the polar opposite of this. The condensation reaction forms a glycosidic link between the joining sugars in dehydration synthesis, and water is liberated in the process. Hydrolysis, which utilises water molecules to dissolve the glycosidic bond and release the sugar components, is used in saccharification.
Maltose is seldom seen in food, although it is made from partly hydrolyzed starch (e.g. maltodextrin and corn syrup). Maltose is produced by the digestion of starch. Amylase is an enzyme found in the saliva and pancreatic juice of humans that breaks down starch into simpler carbs like maltose.
Maltose, on the other hand, is poorly absorbed by the human small intestine. It must be broken down further into its saccharide constituents before being taken up by enterocytes, then into the circulation, and lastly into the cells of other tissues such as the liver, kidney, muscles, the brain, adipose, and so on. Maltose is hydrolyzed and broken down into monosaccharide molecules.
Maltase is the enzyme that aids in this process. Maltose is converted to two glucose units when the link between the two glucose units is broken. Enterocytes (intestinal cells) may now absorb the liberated glucose molecules, which are subsequently released into the circulation and taken up by other cells.
Metabolic Disorders of Maltose
One of the metabolic diseases linked to maltose is maltose intolerance. The enzyme maltase is released from the stomach lining during digestion to catalyse the breakdown of maltose into glucose components.
Undigested maltose is caused by low maltase enzyme activity. When the body is unable to digest maltose, water is drawn into the gut from the rest of the body. This causes diarrhoea. The gut flora metabolises the undigested maltose in the colon.
Bloating and discomfort happen as a result of this. Human maltose intolerance is exceedingly uncommon. It’s usually linked to a deficiency in sucrase-isomaltase enzymes.
Biological Importance / Functions of Maltose
Disaccharides in the diet are eaten and digested to produce simple sugars that may be absorbed and metabolised quickly. One of the most common sources of glucose is maltose. Because glucose is primarily employed in energy metabolism, it is a critical nutrient.
The most prevalent type of monosaccharide used by the cell to generate ATP is glucose, which is phosphorylated at the substrate level (glycolysis) and/or oxidatively phosphorylated (involving redox reactions and chemiosmosis).
Maltose is converted into starch. In the sense that they are both made up of glucose units, starch and maltose are physically identical. Starch, on the other hand, is a glucose polymer, whereas maltose is a glucose disaccharide.
Maltose, on the other hand, is generally produced by the digestion (or hydrolysis) of starch. Through the catalytic activity of beta-amylase, two glucose units (i.e. maltose) from starch are cleaved. This is what happens when seeds germinate, for example.
Maltose is a sweetener, a nutrition in baby formula, and a component of bacteriological culture medium. It’s also utilised in baked goods. When carbon dioxide is generated and released during the conversion of starch into maltose by reacting the starch with enzymes, it causes bread dough to rise. It is less sweet than other sugars when used as a sweetener. Maltose, on the other hand, is not recommended for diabetics because to its high glycemic index.
Maltose Citations
- An integrated transport mechanism of the maltose ABC importer. Res Microbiol . Nov-Dec 2019;170(8):321-337.
- Lactose, Maltose, and Sucrose in Health and Disease. Mol Nutr Food Res . 2020 Apr;64(8):e1901082.
- The History of Maltose-active Disaccharidases. J Pediatr Gastroenterol Nutr . 2018 Jun;66 Suppl 3:S4-S6.
- Figures are created with BioRender.com